
Global Atopic Dermatitis Drugs Market Size, Trends & Analysis - Forecasts to 2028 By Drug Class (Corticosteroids, Calcineurin Inhibitors, PDE4 Inhibitors, Biologics, and Others), By Route of Administration (Topical, Injectable, and Oral), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies), and By Region (North America, Asia Pacific, Central & South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
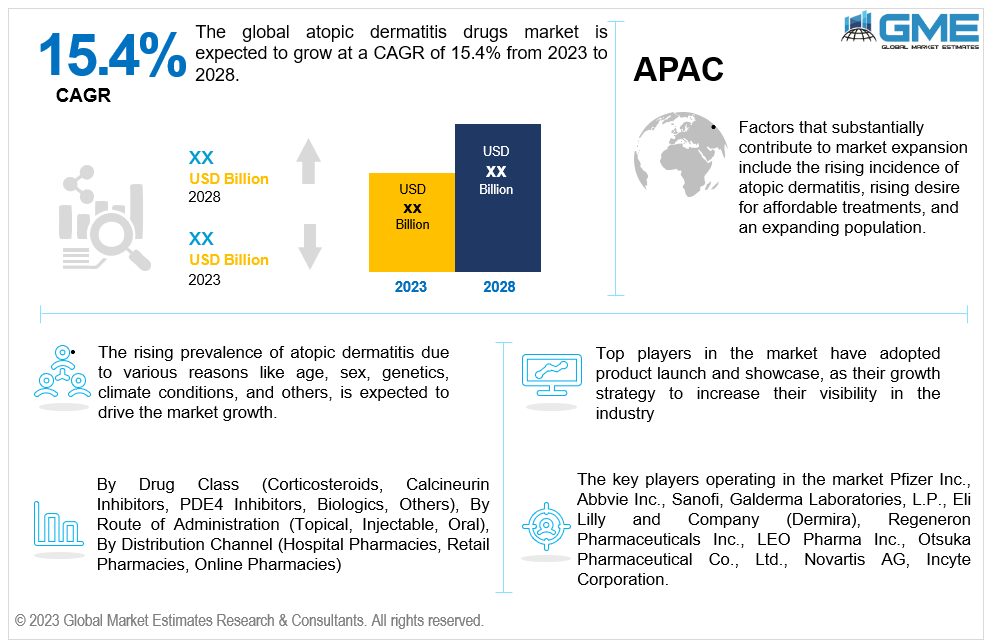
The global atopic dermatitis drugs market is expected to grow at a CAGR of 15.4% from 2023 to 2028. Atopic dermatitis is one of the varieties of dermatitis and is a chronic disorder. Skin with atopic dermatitis is rough, dry, red, and itchy. In the case of atopic dermatitis, skin barrier function is damaged. The skin becomes more sensitive and drier and is prone to infection. Drugs like atopic eczema steroids are used to improve the condition. Atopic dermatitis affects 10% to 20% of infants and is common among them. As kids age, approximately half outgrow the illness or see a significant improvement. Equally affecting men and women, atopic dermatitis is more prevalent in those with a personal or familial history of asthma, environmental allergies, and/or food allergies. Atopic dermatitis can manifest anywhere on the skin. It often appears on the hands, neck, inner elbows, ankles, knees, feet, and the area behind the eyes in adults and teenagers. The atopic dermatitis drugs market was valued at USD 6 billion in 2021 and is expected to generate significant revenue during the forecast period.
The rising prevalence of atopic dermatitis due to various reasons like age, sex, genetics, climate conditions, and others, is expected to drive the market growth. As the atopic dermatitis industry is lucrative, market players are increasingly investing in R&D to develop novel therapies. Leading businesses predict that the increasing number of medications that have advanced to the final phases of clinical trials after demonstrating promising findings will contribute to the market growth during the forecast period. In addition, biologics' availability to treat atopic dermatitis and a rise in product approvals would spur market expansion over the forecast period.
However, allergic reactions to off-label treatments and the expiration of product patents could hinder the market's growth during the forecast period. The market growth is also expected to be hampered by inadequate reimbursement practices in underdeveloped nations worldwide.
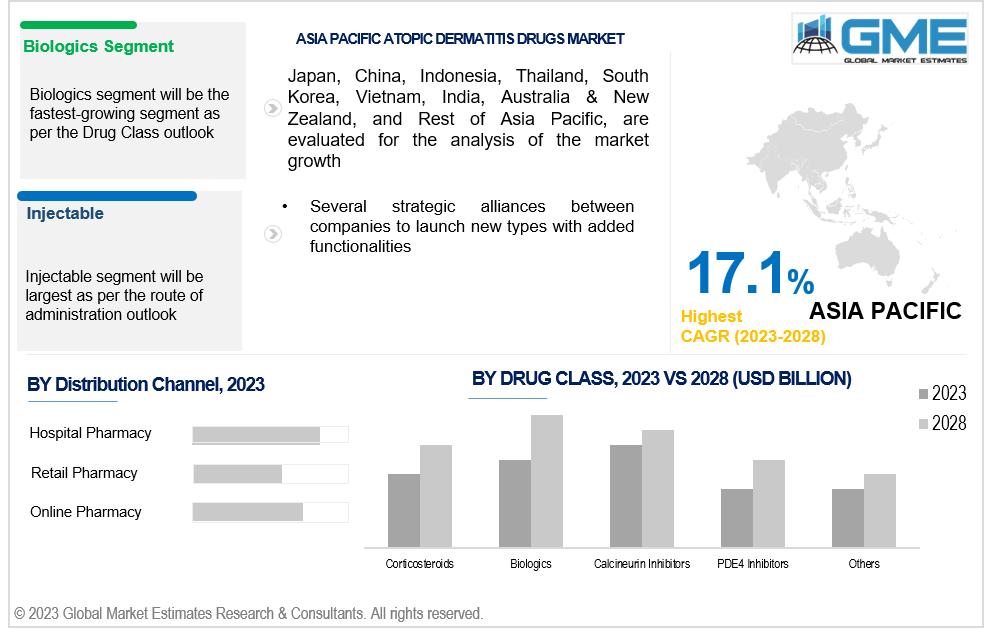
Based on drug class, the global atopic dermatitis drugs market is segmented into corticosteroids, calcineurin inhibitors, pde4 inhibitors, biologics, and others. The biologics segment is expected to be the largest segment in the forecast period due to rising knowledge about the disease's treatable conditions and a rise in the number of government programmes to offer better and more economical care. Moreover, new product launches and a rise in the use of biological therapies in various geographical areas, also contribute to the segment growth.
Based on route of administration the market is segmented into topical, injectable, and oral. Injectable segment is expected to be the largest segment over the forecast period.
Based on distribution channel the market is segmented into hospital pharmacies, retail pharmacies, online pharmacies and others. The retail pharmacy segment is expected to be the largest market segment between 2023 and 2028. One of the main factors influencing the expansion of the retail pharmacy segment is the high incidence of atopic dermatitis, rising medicine price, growing income levels among lower and middle-class families, and drug approvals. Also, an increasing number of initiatives from various governmental agencies to improve the infrastructure of hospitals and pharmacies is anticipated to accelerate the expansion of the hospital pharmacies segment.
North America is analyzed to be the largest region in the global atopic dermatitis drugs market in the forecast period, owing to increasing incidence of atopic dermatitis, well-developed healthcare system, increasing treatment awareness, and advancements in the atopic dermatitis industry. The region's continued dominance is largely due to favourable reimbursement policies and a robust R&D infrastructure.
Asia Pacific is also analyzed to be the fastest growing region in the global atopic dermatitis drugs market with a CAGR of over 17.1%. Moreover, factors that substantially contribute to market expansion include the rising incidence of atopic dermatitis and increasing need for affordable treatments.
The key players operating in the market Pfizer Inc., Abbvie Inc., Sanofi, Galderma Laboratories, L.P., Eli Lilly and Company (Dermira), Regeneron Pharmaceuticals Inc., LEO Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Novartis AG, Incyte Corporation. The global atopic dermatitis drugs market has observed several strategic alliances between companies to launch new products with added functionalities and maintain revenue share & profitability. Organic and inorganic growth strategies adopted by small players have been the highlight of this market.
Please note: This is not an exhaustive list of companies profiled in the report.
For instance, Galderma released phase 2b data in individuals with uncontrolled atopic dermatitis in April 2021 for their unlicensed medication Nemolizumab.
Moreover, in June 2022, Sanofi announced that its Dupixent (dupilumab) was approved by the U.S. FDA for use in children with atopic dermatitis between the ages of 6 months and 5.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
2.4 Data Metrics on Feed Stocks
3 GLOBAL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL ATOPIC DERMATITIS DRUGS MARKET, BY DRUG CLASS
4.1 Introduction
4.2 Atopic Dermatitis Drugs Market: Type Scope Key Takeaways
4.3 Revenue Growth Analysis, 2022 & 2028
4.4 Adverse Drug Reaction Capture (ADR)
4.4.1 Adverse Drug Reaction Capture (ADR) Market Estimates and Forecast, 2020-2028 (USD Million)
4.5 Corticosteroids
4.5.1 CorticosteroidsMarket Estimates and Forecast, 2020-2028 (USD Million)
4.6 Calcineurin Inhibitors
4.7.1 Calcineurin InhibitorsMarket Estimates and Forecast, 2020-2028 (USD Million)
4.7 PDE4 Inhibitors
4.7.1 PDE4 Inhibitors Market Estimates and Forecast, 2020-2028 (USD Million)
4.8 Biologics
4.8.1 Biologics Market Estimates and Forecast, 2020-2028 (USD Million)
4.9 Others
4.9.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
5 GLOBAL ATOPIC DERMATITIS DRUGS MARKET, BY ROUTE OF ADMINISTRATION
5.1 Introduction
5.2 Atopic Dermatitis Drugs Market: Route of Administration Scope Key Takeaways
5.3 Revenue Growth Analysis, 2022 & 2028
5.4 Topical
5.4.1 TopicalMarket Estimates and Forecast, 2020-2028 (USD Million)
5.5.1 Injectable Market Estimates and Forecast, 2020-2028 (USD Million)
5.5 Oral
5.5.1 Oral Market Estimates and Forecast, 2020-2028 (USD Million)
6 GLOBAL ATOPIC DERMATITIS DRUGS MARKET, BY DISTRIBUTION CHANNEL
6.1 Introduction
6.2 Atopic Dermatitis Drugs Market: Distribution Channel Scope Key Takeaways
6.3 Revenue Growth Analysis, 2022 & 2028
6.4 Hospital Pharmacy
6.4.1 Hospital Pharmacy Market Estimates and Forecast, 2020-2028 (USD Million)
6.5 Retail Pharmacy
6.5.1 Retail Pharmacy Market Estimates and Forecast, 2020-2028 (USD Million)
6.6 Online Pharmacy
6.7.1 Online Pharmacy Market Estimates and Forecast, 2020-2028 (USD Million)
7 GLOBAL ATOPIC DERMATITIS DRUGS MARKET, BY REGION
7.1 Introduction
7.2 North America Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.1 By Drug Class
7.2.2 By Route of Administration
7.2.3 By Distribution Channel
7.2.4 By Country
7.2.4.1 U.S. Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.4.1.1 By Drug Class
7.2.4.1.2 By Route of Administration
7.2.4.1.3 By Distribution Channel
7.2.4.2 Canada Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.4.2.1 By Drug Class
7.2.4.2.2 By Route of Administration
7.2.4.2.3 By Distribution Channel
7.2.4.3 Mexico Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.4.3.1 By Drug Class
7.2.4.3.2 By Route of Administration
7.2.4.3.3 By Distribution Channel
7.3 Europe Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.1 By Drug Class
7.3.2 By Route of Administration
7.3.3 By Distribution Channel
7.3.4 By Country
7.3.4.1 Germany Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.1.1 By Drug Class
7.3.4.1.2 By Route of Administration
7.3.4.1.3 By Distribution Channel
7.3.4.2 U.K. Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.2.1 By Drug Class
7.3.4.2.2 By Route of Administration
7.3.4.2.3 By Distribution Channel
7.3.4.3 France Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.3.1 By Drug Class
7.3.4.3.2 By Route of Administration
7.3.4.3.3 By Distribution Channel
7.3.4.4 Italy Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.4.1 By Drug Class
7.3.4.4.2 By Route of Administration
7.2.4.4.3 By Distribution Channel
7.3.4.5 Spain Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.5.1 By Drug Class
7.3.4.5.2 By Route of Administration
7.2.4.5.3 By Distribution Channel
7.3.4.6 Netherlands Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.7.1 By Drug Class
7.3.4.7.2 By Route of Administration
7.2.4.7.3 By Distribution Channel
7.3.4.7 Rest of Europe Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.7.1 By Drug Class
7.3.4.7.2 By Route of Administration
7.2.4.7.3 By Distribution Channel
7.4 Asia Pacific Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.1 By Drug Class
7.4.2 By Route of Administration
7.4.3 By Distribution Channel
7.4.4 By Country
7.4.4.1 China Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.1.1 By Drug Class
7.4.4.1.2 By Route of Administration
7.4.4.1.3 By Distribution Channel
7.4.4.2 Japan Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.2.1 By Drug Class
7.4.4.2.2 By Route of Administration
7.4.4.2.3 By Distribution Channel
7.4.4.3 India Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.3.1 By Drug Class
7.4.4.3.2 By Route of Administration
7.4.4.3.3 By Distribution Channel
7.4.4.4 South Korea Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.4.1 By Drug Class
7.4.4.4.2 By Route of Administration
7.4.4.4.3 By Distribution Channel
7.4.4.5 Singapore Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.5.1 By Drug Class
7.4.4.5.2 By Route of Administration
7.4.4.5.3 By Distribution Channel
7.4.4.6 Malaysia Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.7.1 By Drug Class
7.4.4.7.2 By Route of Administration
7.4.4.7.3 By Distribution Channel
7.4.4.7 Thailand Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.7.1 By Drug Class
7.4.4.7.2 By Route of Administration
7.4.4.7.3 By Distribution Channel
7.4.4.8 Indonesia Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.8.1 By Drug Class
7.4.4.8.2 By Route of Administration
7.4.4.8.3 By Distribution Channel
7.4.4.9 Vietnam Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.9.1 By Drug Class
7.4.4.9.2 By Route of Administration
7.4.4.9.3 By Distribution Channel
7.4.4.10 Taiwan Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.10.1 By Drug Class
7.4.4.10.2 By Route of Administration
7.4.4.10.3 By Distribution Channel
7.4.4.11 Rest of Asia Pacific Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.11.1 By Drug Class
7.4.4.11.2 By Route of Administration
7.4.4.11.3 By Distribution Channel
7.5 Middle East and Africa Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.1 By Drug Class
7.5.2 By Route of Administration
7.5.3 By Distribution Channel
7.5.4 By Country
7.5.4.1 Saudi Arabia Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.1.1 By Drug Class
7.5.4.1.2 By Route of Administration
7.5.4.1.3 By Distribution Channel
7.5.4.2 U.A.E. Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.2.1 By Drug Class
7.5.4.2.2 By Route of Administration
7.5.4.2.3 By Distribution Channel
7.5.4.3 Israel Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.3.1 By Drug Class
7.5.4.3.2 By Route of Administration
7.5.4.3.3 By Distribution Channel
7.5.4.4 South Africa Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.4.1 By Drug Class
7.5.4.4.2 By Route of Administration
7.5.4.4.3 By Distribution Channel
7.5.4.5 Rest of Middle East and Africa Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.5.1 By Drug Class
7.5.4.5.2 By Route of Administration
7.5.4.5.2 By Distribution Channel
7.6 Central & South America Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.1 By Drug Class
7.7.2 By Route of Administration
7.7.3 By Distribution Channel
7.7.4 By Country
7.7.4.1 Brazil Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.1.1 By Drug Class
7.7.4.1.2 By Route of Administration
7.7.4.1.3 By Distribution Channel
7.7.4.2 Argentina Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.2.1 By Drug Class
7.7.4.2.2 By Route of Administration
7.7.4.2.3 By Distribution Channel
7.7.4.3 Chile Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.3.1 By Drug Class
7.7.4.3.2 By Route of Administration
7.7.4.3.3 By Distribution Channel
7.7.4.4 Rest of Central & South America Atopic Dermatitis Drugs Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.4.1 By Drug Class
7.7.4.4.2 By Route of Administration
7.7.4.4.3 By Distribution Channel
8 COMPETITIVE LANDCAPE
8.1 Company Market Share Analysis
8.2 Four Quadrant Positioning Matrix
8.2.1 Market Leaders
8.2.2 Market Visionaries
8.2.3 Market Challengers
8.2.4 Niche Market Players
8.3 Vendor Landscape
8.3.1 North America
8.3.2 Europe
8.3.3 Asia Pacific
8.3.4 Rest of the World
8.4 Company Profiles
8.4.1 Pfizer Inc.
8.4.1.1 Business Description & Financial Analysis
8.4.1.2 SWOT Analysis
8.4.1.3 Products & Services Offered
8.4.1.4 Strategic Alliances between Business Partners
8.4.2 Sanofi
8.4.2.1 Business Description & Financial Analysis
8.4.2.2 SWOT Analysis
8.4.2.3 Products & Services Offered
8.4.2.4 Strategic Alliances between Business Partners
8.4.3 AbbVie Inc
8.4.3.1 Business Description & Financial Analysis
8.4.3.2 SWOT Analysis
8.4.3.3 Products & Services Offered
8.4.3.4 Strategic Alliances between Business Partners
8.4.4 GALDERMA LABORATORIES, L.P.
8.4.4.1 Business Description & Financial Analysis
8.4.4.2 SWOT Analysis
8.4.4.3 Products & Services Offered
8.4.4.4 Strategic Alliances between Business Partners
8.4.5 Eli Lilly and Company (Dermira)
8.4.5.1 Business Description & Financial Analysis
8.4.5.2 SWOT Analysis
8.4.5.3 Products & Services Offered
8.4.5.4 Strategic Alliances between Business Partners
8.4.6 REGENERON PHARMACEUTICALS INC.
8.4.7.1 Business Description & Financial Analysis
8.4.7.2 SWOT Analysis
8.4.7.3 Products & Services Offered
8.4.7.4 Strategic Alliances between Business Partners
8.4.7 Leo Pharma Inc.
8.4.7.1 Business Description & Financial Analysis
8.4.7.2 SWOT Analysis
8.4.7.3 Products & Services Offered
8.4.8.4 Strategic Alliances between Business Partners
8.4.8 Otsuka Pharmaceutical Co., Ltd.
8.4.8.1 Business Description & Financial Analysis
8.4.8.2 SWOT Analysis
8.4.8.3 Products & Services Offered
8.4.8.4 Strategic Alliances between Business Partners
8.4.9 Novartis AG
8.4.9.1 Business Description & Financial Analysis
8.4.9.2 SWOT Analysis
8.4.9.3 Products & Services Offered
8.4.9.4 Strategic Alliances between Business Partners
8.4.10 Incyte Corporation
8.4.10.1 Business Description & Financial Analysis
8.4.10.2 SWOT Analysis
8.4.10.3 Products & Services Offered
8.4.10.4 Strategic Alliances between Business Partners
8.4.11 Other Companies
8.4.11.1 Business Description & Financial Analysis
8.4.11.2 SWOT Analysis
8.4.11.3 Products & Services Offered
8.4.11.4 Strategic Alliances between Business Partners
9 RESEARCH METHODOLOGY
9.1 Market Introduction
9.1.1 Market Definition
9.1.2 Market Scope & SegAtopic Dermatitis Drugs Isolatestation
9.2 Information ProcureAtopic Dermatitis Drugs Isolatest
9.2.1 Secondary Research
9.2.1.1 Purchased Databases
9.2.1.2 GMEs Internal Data Repository
9.2.1.3 Secondary Resources & Third Party Perspectives
9.2.1.4 Company Information Sources
9.2.2 Primary Research
9.2.2.1 Various Types of Respondents for Primary Interviews
9.2.2.2 Number of Interviews Conducted throughout the Research Process
9.2.2.3 Primary Stakeholders
9.2.2.4 Discussion Guide for Primary Participants
9.2.3 Expert Panels
9.2.3.1 Expert Panels Across 30+ Industry
9.2.4 Paid Local Experts
9.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
9.3 Market Estimation
9.3.1 Top-Down Approach
9.3.1.1 Macro-Economic Indicators Considered
9.3.1.2 Micro-Economic Indicators Considered
9.3.2 Bottom Up Approach
9.3.2.1 Company Share Analysis Approach
9.3.2.2 Estimation of Potential Product Distribution Channel
9.4 Data Triangulation
9.4.1 Data Collection
9.4.2 Time Series, Cross Sectional & Panel Data Analysis
9.4.3 Cluster Analysis
9.5 Analysis and Output
9.5.1 Inhouse AI Based Real Time Analytics Tool
9.5.2 Output From Desk & Primary Research
9.6 Research Assumptions & Limitations
9.7.1 Research Assumptions
9.7.2 Research Limitations
LIST OF TABLES
1 Global Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Mllion)
2 CorticosteroidsMarket, By Region, 2020-2028 (USD Mllion)
3 Calcineurin InhibitorsMarket, By Region, 2020-2028 (USD Mllion)
4 PDE4 Inhibitors Market, By Region, 2020-2028 (USD Mllion)
5 Biologics, By Region, 2020-2028 (USD Mllion)
6 Others, By Region, 2020-2028 (USD Mllion)
7 Global Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Mllion)
8 TopicalMarket, By Region, 2020-2028 (USD Mllion)
9 Injectable Market, By Region, 2020-2028 (USD Mllion)
10 Oral Market, By Region, 2020-2028 (USD Mllion)
11 Global Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Mllion)
12 Hospital Pharmacy Market, By Region, 2020-2028 (USD Mllion)
13 Retail Pharmacy Market, By Region, 2020-2028 (USD Mllion)
14 Online Pharmacy Market, By Region, 2020-2028 (USD Mllion)
15 Regional Analysis, 2020-2028 (USD Mllion)
16 North America Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
17 North America Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
18 North America Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
19 North America Atopic Dermatitis Drugs Market, By Country, 2020-2028 (USD Million)
20 U.S Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
21 U.S Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
22 U.S Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
23 Canada Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
24 Canada Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
25 Canada Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
26 Mexico Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
27 Mexico Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
28 Mexico Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
29 Europe Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
30 Europe Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
31 Europe Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
32 Germany Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
33 Germany Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
34 Germany Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
35 UK Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
36 UK Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
37 UK Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
38 France Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
39 France Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
40 France Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
41 Italy Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
42 Italy Atopic Dermatitis Drugs Market, By T Route of Administration Type, 2020-2028 (USD Million)
43 Italy Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
44 Spain Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
45 Spain Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
46 Spain Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
47 Rest Of Europe Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
48 Rest Of Europe Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
49 Rest of Europe Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
50 Asia Pacific Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
51 Asia Pacific Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
52 Asia Pacific Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
53 Asia Pacific Atopic Dermatitis Drugs Market, By Country, 2020-2028 (USD Million)
54 China Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
55 China Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
56 China Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
57 India Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
58 India Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
59 India Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
60 Japan Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
61 Japan Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
62 Japan Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
63 South Korea Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
64 South Korea Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
65 South Korea Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
66 Middle East and Africa Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
67 Middle East and Africa Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
68 Middle East and Africa Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
69 Middle East and Africa Atopic Dermatitis Drugs Market, By Country, 2020-2028 (USD Million)
70 Saudi Arabia Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
71 Saudi Arabia Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
72 Saudi Arabia Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
73 UAE Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
74 UAE Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
75 UAE Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
76 Central & South America Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
77 Central & South America Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
78 Central & South America Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
79 Central & South America Atopic Dermatitis Drugs Market, By Country, 2020-2028 (USD Million)
80 Brazil Atopic Dermatitis Drugs Market, By Drug Class, 2020-2028 (USD Million)
81 Brazil Atopic Dermatitis Drugs Market, By Route of Administration, 2020-2028 (USD Million)
82 Brazil Atopic Dermatitis Drugs Market, By Distribution Channel, 2020-2028 (USD Million)
83 Pfizer Inc.: Products & Services Offering
84 Sanofi: Products & Services Offering
85 AbbVie Inc: Products & Services Offering
86 GALDERMA LABORATORIES, L.P.: Products & Services Offering
87 Eli Lilly and Company (Dermira): Products & Services Offering
88 REGENERON PHARMACEUTICALS INC.: Products & Services Offering
89 Leo Pharma Inc.: Products & Services Offering
90 Otsuka Pharmaceutical Co., Ltd.: Products & Services Offering
91 Novartis AG : Products & Services Offering
92 Incyte Corporation: Products & Services Offering
93 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global Atopic Dermatitis Drugs Market Overview
2 Global Atopic Dermatitis Drugs Market Value From 2020-2028 (USD Mllion)
3 Global Atopic Dermatitis Drugs Market Share, By Drug Class (2022)
4 Global Atopic Dermatitis Drugs Market Share, By Route of Administration (2022)
5 Global Atopic Dermatitis Drugs Market Share, By Distribution Channel (2022)
6 Global Atopic Dermatitis Drugs Market, By Region (Asia Pacific Market)
7 Technological Trends In Global Atopic Dermatitis Drugs Market
8 Four Quadrant Competitor Positioning Matrix
9 Impact Of Macro & Micro Indicators On The Market
10 Impact Of Key Drivers On The Global Atopic Dermatitis Drugs Market
11 Impact Of Challenges On The Global Atopic Dermatitis Drugs Market
12 Porter’s Five Forces Analysis
13 Global Atopic Dermatitis Drugs Market: By Drug Class Scope Key Takeaways
14 Global Atopic Dermatitis Drugs Market, By Drug Class Segment: Revenue Growth Analysis
15 Corticosteroids Market, By Region, 2020-2028 (USD Mllion)
16 Calcineurin Inhibitors Market, By Region, 2020-2028 (USD Mllion)
17 PDE4 Inhibitors Market, By Region, 2020-2028 (USD Mllion)
18 Biologics Market, By Region, 2020-2028 (USD Mllion)
19 Others Market, By Region, 2020-2028 (USD Mllion)
20 Global Atopic Dermatitis Drugs Market: By Route of Administration Scope Key Takeaways
21 Global Atopic Dermatitis Drugs Market, By Route of Administration Segment: Revenue Growth Analysis
22 TopicalMarket, By Region, 2020-2028 (USD Mllion)
23 Injectable Market, By Region, 2020-2028 (USD Mllion)
24 Oral Market, By Region, 2020-2028 (USD Mllion)
25 Global Atopic Dermatitis Drugs Market: By Distribution Channel Scope Key Takeaways
26 Global Atopic Dermatitis Drugs Market, By Distribution Channel Segment: Revenue Growth Analysis
27 Hospital Pharmacy Market, By Region, 2020-2028 (USD Mllion)
28 Retail Pharmacy Market, By Region, 2020-2028 (USD Mllion)
29 Online Pharmacy Market, By Region, 2020-2028 (USD Mllion)
30 Regional Segment: Revenue Growth Analysis
31 Global Atopic Dermatitis Drugs Market: Regional Analysis
32 North America Atopic Dermatitis Drugs Market Overview
33 North America Atopic Dermatitis Drugs Market, By Drug Class
34 North America Atopic Dermatitis Drugs Market, By Route of Administration
35 North America Atopic Dermatitis Drugs Market, By Distribution Channel
36 North America Atopic Dermatitis Drugs Market, By Country
37 U.S. Atopic Dermatitis Drugs Market, By Drug Class
38 U.S. Atopic Dermatitis Drugs Market, By Route of Administration
39 U.S. Atopic Dermatitis Drugs Market, By Distribution Channel
40 Canada Atopic Dermatitis Drugs Market, By Drug Class
41 Canada Atopic Dermatitis Drugs Market, By Route of Administration
42 Canada Atopic Dermatitis Drugs Market, By Distribution Channel
43 Mexico Atopic Dermatitis Drugs Market, By Drug Class
44 Mexico Atopic Dermatitis Drugs Market, By Route of Administration
45 Mexico Atopic Dermatitis Drugs Market, By Distribution Channel
46 Four Quadrant Positioning Matrix
47 Company Market Share Analysis
48 Pfizer Inc.: Company Snapshot
49 Pfizer Inc.: SWOT Analysis
50 Pfizer Inc.: Geographic Presence
51 Sanofi: Company Snapshot
52 Sanofi: SWOT Analysis
53 Sanofi: Geographic Presence
54 AbbVie Inc: Company Snapshot
55 AbbVie Inc: SWOT Analysis
56 AbbVie Inc: Geographic Presence
57 GALDERMA LABORATORIES, L.P.: Company Snapshot
58 GALDERMA LABORATORIES, L.P.: Swot Analysis
59 GALDERMA LABORATORIES, L.P.: Geographic Presence
60 Eli Lilly and Company (Dermira): Company Snapshot
61 Eli Lilly and Company (Dermira): SWOT Analysis
62 Eli Lilly and Company (Dermira): Geographic Presence
63 REGENERON PHARMACEUTICALS INC.: Company Snapshot
64 REGENERON PHARMACEUTICALS INC.: SWOT Analysis
65 REGENERON PHARMACEUTICALS INC.: Geographic Presence
66 Leo Pharma Inc.: Company Snapshot
67 Leo Pharma Inc.: SWOT Analysis
68 Leo Pharma Inc.: Geographic Presence
69 Otsuka Pharmaceutical Co., Ltd.: Company Snapshot
70 Otsuka Pharmaceutical Co., Ltd.: SWOT Analysis
71 Otsuka Pharmaceutical Co., Ltd.: Geographic Presence
72 Novartis AG: Company Snapshot
73 Novartis AG: SWOT Analysis
74 Novartis AG: Geographic Presence
75 Incyte Corporation: Company Snapshot
76 Incyte Corporation: SWOT Analysis
77 Incyte Corporation: Geographic Presence
78 Other Companies: Company Snapshot
79 Other Companies: SWOT Analysis
80 Other Companies: Geographic Presence
The Global Atopic Dermatitis Drugs Market has been studied from the year 2019 till 2028. However, the CAGR provided in the report is from the year 2023 to 2028. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Atopic Dermatitis Drugs Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Frequently Asked Questions
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS