
Global In Vitro Diagnostics Quality Control Market Size, Trends & Analysis - Forecasts to 2029 By Application (Immunochemistry, Hematology, Clinical Chemistry, Molecular Diagnostics, Coagulation, Microbiology, and Others), By Type (Quality Controls, Data Management Solutions, and Quality Assurance Services), By End-use (Home Care, Laboratory, Hospitals, and Others), and By Region (North America, Asia Pacific Central and South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
The global vitro diagnostics quality control market is projected to grow at a CAGR of 4.3% from 2024 to 2029.
The growing utilization of IVD tests for disease diagnosis and management is fuelling the growth of the global vitro diagnostics quality control market. As healthcare providers and laboratories perform more IVD tests, the demand for quality control solutions rises.
The increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular conditions, along with infectious diseases, necessitates accurate and reliable diagnostic testing, boosting the need for quality control. As per the World Health Organization's 2019 report, respiratory diseases stand as the primary global cause of mortality, resulting in approximately 4.0 million deaths annually.
Patient safety and the need for accurate test results are paramount. Errors in diagnostic tests can have serious consequences, prompting laboratories and healthcare facilities to invest in quality control to minimize mistakes.
The aging population in many countries leads to a higher incidence of age-related diseases, increasing the demand for diagnostic tests and quality control measures. According to the World Health Organization (WHO), in 2030, it is anticipated that one-sixth of the world's population will be 60 years old or older, marking an increase from 1 billion in 2020 to 1.4 billion.
Immunochemistry segment is expected to hold the largest share of the market. The primary applications of immunochemistry involve identifying infectious microorganisms like influenza, bacteria, and fungi through the detection of their toxins and surface antigens. The increasing adoption of multi-analyte controls for conducting immunoassay experiments in laboratory settings is a significant driver for the growth of the in vitro diagnostics (IVD) quality control market.
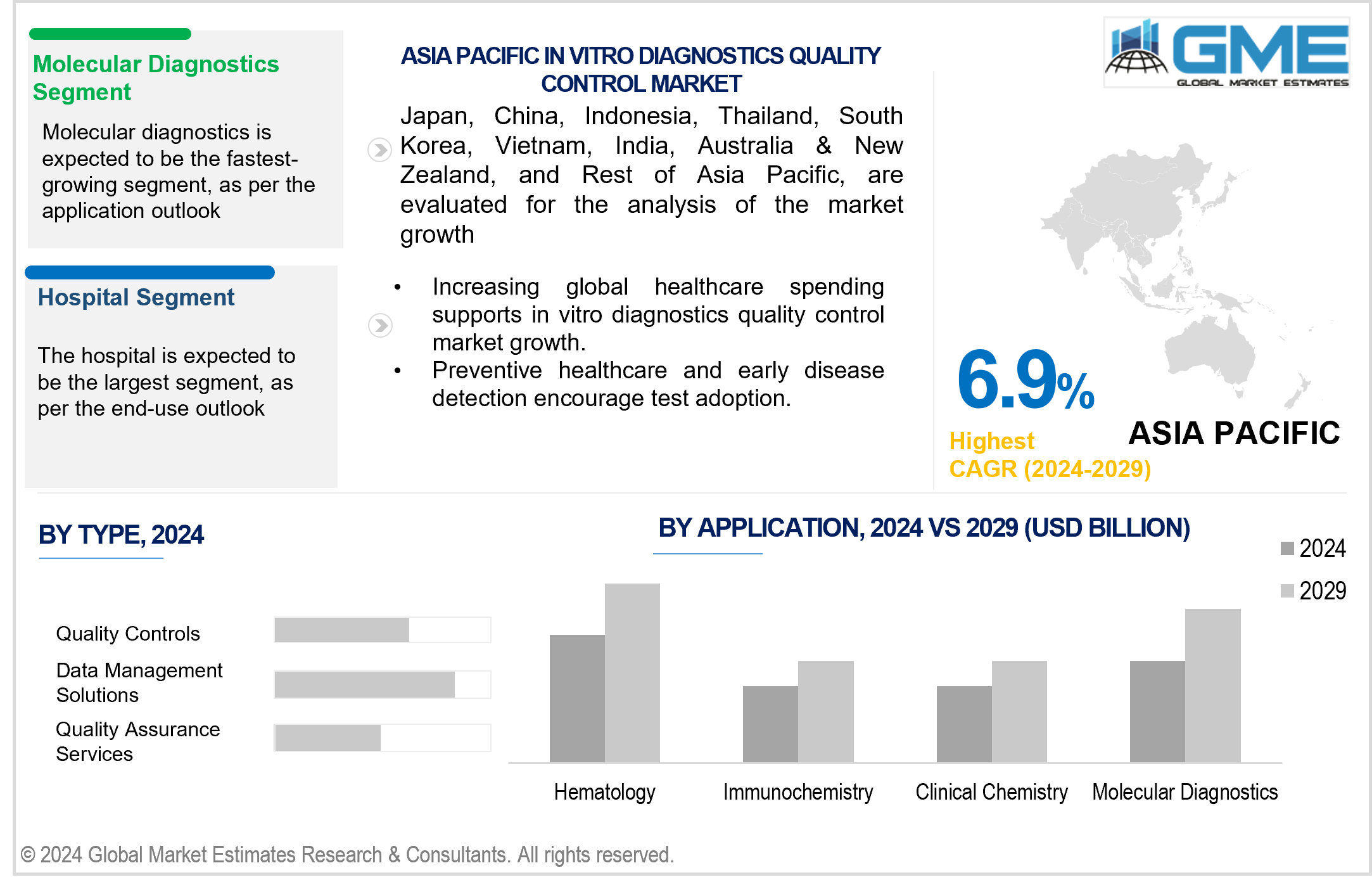
Molecular diagnostics segment is expected to be the fastest-growing segment in the market. Molecular diagnostics includes a variety of complex tests utilized for the medical detection and diagnosis of significant viral, bacterial, and parasitic infections. The adoption of advanced technologies like PCR and next-generation sequencing has addressed the limitations of traditional systems, including issues related to speed, sensitivity, and accuracy.
Quality control segment is expected to hold the largest share of the market. Quality control checks are carried out during various stages, including instrument installation, switching reagent lots, when readings fall outside the expected range, and after servicing.
Various regulatory bodies, including the American Association for Laboratory Accreditation (A2LA), Clinical Laboratory Improvement Amendments (CLIA), and the European Communities Confederation of Clinical Chemistry and Laboratory Medicine (EC4), have played a role in driving up the need for quality control in In Vitro Diagnostics (IVD).
The homecare segment is anticipated to be the fastest-growing segment in the market during the forecast period. Home care products encompass a range of items such as blood glucose monitoring devices for individuals with diabetes, pregnancy detection kits, urine analysers, haemoglobin sensors, and hormone home testing kits. These devices carry a significant risk because they are used by individuals without specialized medical training. To ensure patient safety, quality control measures are implemented through both pre- and post-marketing quality assurance and quality control processes for these devices.
The hospital segment is expected to hold the largest share of the market. This growth can be attributed to the increasing frequency of hospital admissions and the reliance of healthcare practitioners on clinical assessments to guide treatment choices. Due to the high volume of procedures conducted in this department, it is essential to regularly monitor the tests and instruments used.
In order to fulfill quality control requirements, third-party companies like Bio-Rad and Randox have entered collaborations with hospitals, supplying them with the necessary reagents.
North America is expected to be the largest region in the market. The primary reasons boosting the market growth in this region is the presence of the U.S. FDA (Food and Drug Administration) and numerous accredited diagnostic laboratories, bolstered by robust quality control regulatory systems. Both the U.S. FDA and the GHTF (Global Harmonization Task Force) jointly oversee and regulate the IVD quality control market in North America.
Asia Pacific is predicted to witness rapid growth during the forecast period. This is due to growing awareness regarding the importance of early and accurate diagnosis. Entities such as the Asia Pacific Federation of Clinical Biochemistry and Laboratory Medicine (APFCB) play an active role in promoting awareness regarding the practicality of In Vitro Diagnostics (IVDs) in the Asia Pacific region. Japan has regulatory bodies such as the Ministry of Health, Labor, and Welfare (MHLW), the Pharmaceuticals and Medical Devices Evaluation Agency (PMDA), and the Ministry of Agriculture and Fisheries and Food, which hold significant responsibilities in supervising and governing the healthcare sector.
Siemens Healthcare GmbH, F. Hoffmann-La Roche Ltd., Alere, Inc., Abbott, Bio-Techne, Hologic, Inc. (Gen-Probe), Qiagen N.V., Bio-Rad Laboratories, Inc., Quidel Corp., and bioMerieux, Inc., among others, are some of the key players operating in the global vitro diagnostics quality control market.
Please note: This is not an exhaustive list of companies profiled in the report.
In May 2023, ZeptoMetrix introduced a new range of quality control items called PROtrol, tailored explicitly for antigen-based diagnostic techniques like the lateral flow immunoassay used in infectious disease testing. These materials oversee and assess the efficiency of antigen assays, ultimately enhancing the overall quality of these tests. This product launch is expected to make the company more appealing to potential partnerships with various diagnostic test manufacturers.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
3 GLOBAL IN VITRO DIAGNOSTICS QUALITY CONTROL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL IN VITRO DIAGNOSTICS QUALITY CONTROL MARKET, BY APPLICATION
4.1 Introduction
4.2 In Vitro Diagnostics Quality Control Market: Application Scope Key Takeaways
4.3 Revenue Growth Analysis, 2023 & 2029
4.4 Immunochemistry
4.4.1 Immunochemistry Market Estimates and Forecast, 2021-2029 (USD Million)
4.5 Hematology
4.5.1 Hematology Market Estimates and Forecast, 2021-2029 (USD Million)
4.6 Clinical Chemistry
4.6.1 Clinical Chemistry Market Estimates and Forecast, 2021-2029 (USD Million)
4.7 Molecular Diagnostics
4.7.1 Molecular Diagnostics Market Estimates and Forecast, 2021-2029 (USD Million)
4.8 Coagulation
4.8.1 Coagulation Market Estimates and Forecast, 2021-2029 (USD Million)
4.9 Microbiology
4.9.1 Microbiology Market Estimates and Forecast, 2021-2029 (USD Million)
4.10 Others
4.10.1 Others Market Estimates and Forecast, 2021-2029 (USD Million)
5 GLOBAL IN VITRO DIAGNOSTICS QUALITY CONTROL MARKET, BY TYPE
5.1 Introduction
5.2 In Vitro Diagnostics Quality Control Market: Type Scope Key Takeaways
5.3 Revenue Growth Analysis, 2023 & 2029
5.4 Quality Controls
5.4.1 Quality Controls Market Estimates and Forecast, 2021-2029 (USD Million)
5.5 Data Management Solutions
5.5.1 Data Management Solutions Market Estimates and Forecast, 2021-2029 (USD Million)
5.6 Quality Assurance Services
5.6.1 Quality Assurance Services Market Estimates and Forecast, 2021-2029 (USD Million)
6 GLOBAL IN VITRO DIAGNOSTICS QUALITY CONTROL MARKET, BY END-USE
6.1 Introduction
6.2 In Vitro Diagnostics Quality Control Market: End-use Scope Key Takeaways
6.3 Revenue Growth Analysis, 2023 & 2029
6.4 Home Care
6.4.1 Home Care Market Estimates and Forecast, 2021-2029 (USD Million)
6.5 Laboratory
6.5.1 Laboratory Market Estimates and Forecast, 2021-2029 (USD Million)
6.6 Hospitals
6.6.1 Hospitals Market Estimates and Forecast, 2021-2029 (USD Million)
6.7 Others
6.7.1 Others Market Estimates and Forecast, 2021-2029 (USD Million)
7 GLOBAL IN VITRO DIAGNOSTICS QUALITY CONTROL MARKET, BY REGION
7.1 Introduction
7.2 North America In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.2.1 By Application
7.2.2 By Type
7.2.3 By End-use
7.2.4 By Country
7.2.4.1 U.S. In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.2.4.1.1 By Application
7.2.4.1.2 By Type
7.2.4.1.3 By End-use
7.2.4.2 Canada In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.2.4.2.1 By Application
7.2.4.2.2 By Type
7.2.4.2.3 By End-use
7.2.4.3 Mexico In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.2.4.3.1 By Application
7.2.4.3.2 By Type
7.2.4.3.3 By End-use
7.3 Europe In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.1 By Application
7.3.2 By Type
7.3.3 By End-use
7.3.4 By Country
7.3.4.1 Germany In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.1.1 By Application
7.3.4.1.2 By Type
7.3.4.1.3 By End-use
7.3.4.2 U.K. In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.2.1 By Application
7.3.4.2.2 By Type
7.3.4.2.3 By End-use
7.3.4.3 France In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.3.1 By Application
7.3.4.3.2 By Type
7.3.4.3.3 By End-use
7.3.4.4 Italy In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.4.1 By Application
7.3.4.4.2 By Type
7.2.4.4.3 By End-use
7.3.4.5 Spain In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.5.1 By Application
7.3.4.5.2 By Type
7.2.4.5.3 By End-use
7.3.4.6 Netherlands In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.6.1 By Application
7.3.4.6.2 By Type
7.2.4.6.3 By End-use
7.3.4.7 Rest of Europe In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.3.4.7.1 By Application
7.3.4.7.2 By Type
7.2.4.7.3 By End-use
7.4 Asia Pacific In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.1 By Application
7.4.2 By Type
7.4.3 By End-use
7.4.4 By Country
7.4.4.1 China In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.1.1 By Application
7.4.4.1.2 By Type
7.4.4.1.3 By End-use
7.4.4.2 Japan In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.2.1 By Application
7.4.4.2.2 By Type
7.4.4.2.3 By End-use
7.4.4.3 India In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.3.1 By Application
7.4.4.3.2 By Type
7.4.4.3.3 By End-use
7.4.4.4 South Korea In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.4.1 By Application
7.4.4.4.2 By Type
7.4.4.4.3 By End-use
7.4.4.5 Singapore In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.5.1 By Application
7.4.4.5.2 By Type
7.4.4.5.3 By End-use
7.4.4.6 Malaysia In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.6.1 By Application
7.4.4.6.2 By Type
7.4.4.6.3 By End-use
7.4.4.7 Thailand In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.7.1 By Application
7.4.4.7.2 By Type
7.4.4.7.3 By End-use
7.4.4.8 Indonesia In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.8.1 By Application
7.4.4.8.2 By Type
7.4.4.8.3 By End-use
7.4.4.9 Vietnam In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.9.1 By Application
7.4.4.9.2 By Type
7.4.4.9.3 By End-use
7.4.4.10 Taiwan In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.10.1 By Application
7.4.4.10.2 By Type
7.4.4.10.3 By End-use
7.4.4.11 Rest of Asia Pacific In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.4.4.11.1 By Application
7.4.4.11.2 By Type
7.4.4.11.3 By End-use
7.5 Middle East and Africa In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.5.1 By Application
7.5.2 By Type
7.5.3 By End-use
7.5.4 By Country
7.5.4.1 Saudi Arabia In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.5.4.1.1 By Application
7.5.4.1.2 By Type
7.5.4.1.3 By End-use
7.5.4.2 U.A.E. In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.5.4.2.1 By Application
7.5.4.2.2 By Type
7.5.4.2.3 By End-use
7.5.4.3 Israel In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.5.4.3.1 By Application
7.5.4.3.2 By Type
7.5.4.3.3 By End-use
7.5.4.4 South Africa In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.5.4.4.1 By Application
7.5.4.4.2 By Type
7.5.4.4.3 By End-use
7.5.4.5 Rest of Middle East and Africa In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.5.4.5.1 By Application
7.5.4.5.2 By Type
7.5.4.5.2 By End-use
7.6 Central and South America In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.6.1 By Application
7.6.2 By Type
7.6.3 By End-use
7.6.4 By Country
7.6.4.1 Brazil In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.6.4.1.1 By Application
7.6.4.1.2 By Type
7.6.4.1.3 By End-use
7.6.4.2 Argentina Eaporative Air Cooler Market Estimates and Forecast, 2021-2029 (USD Million)
7.6.4.2.1 By Application
7.6.4.2.2 By Type
7.6.4.2.3 By End-use
7.6.4.3 Chile In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.6.4.3.1 By Application
7.6.4.3.2 By Type
7.6.4.3.3 By End-use
7.6.4.4 Rest of Central & South America In Vitro Diagnostics Quality Control Market Estimates and Forecast, 2021-2029 (USD Million)
7.6.4.4.1 By Application
7.6.4.4.2 By Type
7.6.4.4.3 By End-use
8 COMPETITIVE LANDCAPE
8.1 Company Market Share Analysis
8.2 Four Quadrant Positioning Matrix
8.2.1 Market Leaders
8.2.2 Market Visionaries
8.2.3 Market Challengers
8.2.4 Niche Market Players
8.3 Vendor Landscape
8.3.1 North America
8.3.2 Europe
8.3.3 Asia Pacific
8.3.4 Rest of the World
8.4 Company Profiles
8.4.1 Siemens Healthcare GmbH
8.4.1.1 Business Description & Financial Analysis
8.4.1.2 SWOT Analysis
8.4.1.3 Products & Services Offered
8.4.1.4 Strategic Alliances between Business Partners
8.4.2 F. Hoffmann-La Roche Ltd.
8.4.2.1 Business Description & Financial Analysis
8.4.2.2 SWOT Analysis
8.4.2.3 Products & Services Offered
8.4.2.4 Strategic Alliances between Business Partners
8.4.3 Alere, Inc.
8.4.3.1 Business Description & Financial Analysis
8.4.3.2 SWOT Analysis
8.4.3.3 Products & Services Offered
8.4.3.4 Strategic Alliances between Business Partners
8.4.4 Abbott
8.4.4.1 Business Description & Financial Analysis
8.4.4.2 SWOT Analysis
8.4.4.3 Products & Services Offered
8.4.4.4 Strategic Alliances between Business Partners
8.4.5 Bio-Techne
8.4.5.1 Business Description & Financial Analysis
8.4.5.2 SWOT Analysis
8.4.5.3 Products & Services Offered
8.4.5.4 Strategic Alliances between Business Partners
8.4.6 Hologic, Inc. (Gen-Probe)
8.4.6.1 Business Description & Financial Analysis
8.4.6.2 SWOT Analysis
8.4.6.3 Products & Services Offered
8.4.6.4 Strategic Alliances between Business Partners
8.4.7 Qiagen N.V.
8.4.7.1 Business Description & Financial Analysis
8.4.7.2 SWOT Analysis
8.4.7.3 Products & Services Offered
8.4.8.4 Strategic Alliances between Business Partners
8.4.8 Bio-Rad Laboratories, Inc.
8.4.8.1 Business Description & Financial Analysis
8.4.8.2 SWOT Analysis
8.4.8.3 Products & Services Offered
8.4.8.4 Strategic Alliances between Business Partners
8.4.9 Quidel Corp.
8.4.9.1 Business Description & Financial Analysis
8.4.9.2 SWOT Analysis
8.4.9.3 Products & Services Offered
8.4.9.4 Strategic Alliances between Business Partners
8.4.10 BioMerieux, Inc.
8.4.10.1 Business Description & Financial Analysis
8.4.10.2 SWOT Analysis
8.4.10.3 Products & Services Offered
8.4.10.4 Strategic Alliances between Business Partners
8.4.11 Other Companies
8.4.11.1 Business Description & Financial Analysis
8.4.11.2 SWOT Analysis
8.4.11.3 Products & Services Offered
8.4.11.4 Strategic Alliances between Business Partners
9 RESEARCH METHODOLOGY
9.1 Market Introduction
9.1.1 Market Definition
9.1.2 Market Scope & Segmentation
9.2 Information Procurement
9.2.1 Secondary Research
9.2.1.1 Purchased Databases
9.2.1.2 GMEs Internal Data Repository
9.2.1.3 Secondary Resources & Third Party Perspectives
9.2.1.4 Company Information Sources
9.2.2 Primary Research
9.2.2.1 Various Types of Respondents for Primary Interviews
9.2.2.2 Number of Interviews Conducted throughout the Research Process
9.2.2.3 Primary Stakeholders
9.2.2.4 Discussion Guide for Primary Participants
9.2.3 Expert Panels
9.2.3.1 Expert Panels Across 30+ Industry
9.2.4 Paid Local Experts
9.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
9.3 Market Estimation
9.3.1 Top-Down Approach
9.3.1.1 Macro-Economic Indicators Considered
9.3.1.2 Micro-Economic Indicators Considered
9.3.2 Bottom Up Approach
9.3.2.1 Company Share Analysis Approach
9.3.2.2 Estimation of Potential Product Sales
9.4 Data Triangulation
9.4.1 Data Collection
9.4.2 Time Series, Cross Sectional & Panel Data Analysis
9.4.3 Cluster Analysis
9.5 Analysis and Output
9.5.1 Inhouse AI Based Real Time Analytics Tool
9.5.2 Output From Desk & Primary Research
9.6 Research Assumptions & Limitations
9.7.1 Research Assumptions
9.7.2 Research Limitations
LIST OF TABLES
1 Global In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Mllion)
2 Immunochemistry Market, By Region, 2021-2029 (USD Mllion)
3 Hematology Market, By Region, 2021-2029 (USD Mllion)
4 Clinical Chemistry Market, By Region, 2021-2029 (USD Mllion)
5 Molecular Diagnostics Market, By Region, 2021-2029 (USD Mllion)
6 Coagulation Market, By Region, 2021-2029 (USD Mllion)
7 Microbiology Market, By Region, 2021-2029 (USD Mllion)
8 Others Market, By Region, 2021-2029 (USD Mllion)
9 Global In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Mllion)
10 Quality Controls Market, By Region, 2021-2029 (USD Mllion)
11 Data Management Solutions Market, By Region, 2021-2029 (USD Mllion)
12 Quality Assurance Services Market, By Region, 2021-2029 (USD Mllion)
13 Global In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Mllion)
14 Home Care Market, By Region, 2021-2029 (USD Mllion)
15 Laboratory Market, By Region, 2021-2029 (USD Mllion)
16 Hospitals Market, By Region, 2021-2029 (USD Mllion)
17 Others Market, By Region, 2021-2029 (USD Mllion)
18 Regional Analysis, 2021-2029 (USD Mllion)
19 North America In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
20 North America In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
21 North America In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
22 North America In Vitro Diagnostics Quality Control Market, By Country, 2021-2029 (USD Million)
23 U.S In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
24 U.S In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
25 U.S In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
26 Canada In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
27 Canada In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
28 Canada In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
29 Mexico In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
30 Mexico In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
31 Mexico In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
32 Europe In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
33 Europe In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
34 Europe In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
35 Europe In Vitro Diagnostics Quality Control Market, By country, 2021-2029 (USD Million)
36 Germany In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
37 Germany In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
38 Germany In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
39 UK In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
40 UK In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
41 UK In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
42 France In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
43 France In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
44 France In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
45 Italy In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
46 Italy In Vitro Diagnostics Quality Control Market, By End Use , 2021-2029 (USD Million)
47 Italy In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
48 Spain In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
49 Spain In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
50 Spain In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
51 Rest Of Europe In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
52 Rest Of Europe In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
53 Rest of Europe In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
54 Asia Pacific In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
55 Asia Pacific In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
56 Asia Pacific In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
57 Asia Pacific In Vitro Diagnostics Quality Control Market, By Country, 2021-2029 (USD Million)
58 China In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
59 China In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
60 China In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
61 India In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
62 India In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
63 India In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
64 Japan In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
65 Japan In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
66 Japan In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
67 South Korea In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
68 South Korea In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
69 South Korea In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
70 Rest of Asia Pacific In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
71 Rest of Asia Pacific In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
72 Rest of Asia Pacific In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
73 Middle East and Africa In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
74 Middle East and Africa In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
75 Middle East and Africa In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
76 Middle East and Africa In Vitro Diagnostics Quality Control Market, By Country, 2021-2029 (USD Million)
77 Saudi Arabia In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
78 Saudi Arabia In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
79 Saudi Arabia In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
80 UAE In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
81 UAE In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
82 UAE In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
83 rest of Middle East and Africa In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
84 Rest of Middle East and Africa In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
85 rest of Middle East and Africa In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
86 Central and South America In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
87 Central and South America In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
88 Central and South America In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
89 Central and South America In Vitro Diagnostics Quality Control Market, By Country, 2021-2029 (USD Million)
90 Brazil In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
91 Brazil In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
92 Brazil In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
93 rest of Central and South America In Vitro Diagnostics Quality Control Market, By Application, 2021-2029 (USD Million)
94 rest of Central and South America In Vitro Diagnostics Quality Control Market, By Type, 2021-2029 (USD Million)
95 rest of Central and South America In Vitro Diagnostics Quality Control Market, By End-use, 2021-2029 (USD Million)
96 Siemens Healthcare GmbH: Products & Services Offering
97 F. Hoffmann-La Roche Ltd.: Products & Services Offering
98 Alere, Inc.: Products & Services Offering
99 Abbott: Products & Services Offering
100 Bio-Techne: Products & Services Offering
101 HOLOGIC, INC. (GEN-PROBE): Products & Services Offering
102 Qiagen N.V. : Products & Services Offering
103 Bio-Rad Laboratories, Inc.: Products & Services Offering
104 Quidel Corp., Inc: Products & Services Offering
105 BioMerieux, Inc.: Products & Services Offering
106 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global In Vitro Diagnostics Quality Control Market Overview
2 Global In Vitro Diagnostics Quality Control Market Value From 2021-2029 (USD Mllion)
3 Global In Vitro Diagnostics Quality Control Market Share, By Application (2023)
4 Global In Vitro Diagnostics Quality Control Market Share, By Type (2023)
5 Global In Vitro Diagnostics Quality Control Market Share, By End-use (2023)
6 Global In Vitro Diagnostics Quality Control Market, By Region (Asia Pacific Market)
7 Technological Trends In Global In Vitro Diagnostics Quality Control Market
8 Four Quadrant Competitor Positioning Matrix
9 Impact Of Macro & Micro Indicators On The Market
10 Impact Of Key Drivers On The Global In Vitro Diagnostics Quality Control Market
11 Impact Of Challenges On The Global In Vitro Diagnostics Quality Control Market
12 Porter’s Five Forces Analysis
13 Global In Vitro Diagnostics Quality Control Market: By Application Scope Key Takeaways
14 Global In Vitro Diagnostics Quality Control Market, By Application Segment: Revenue Growth Analysis
15 Immunochemistry Market, By Region, 2021-2029 (USD Mllion)
16 Hematology Market, By Region, 2021-2029 (USD Mllion)
17 Clinical Chemistry Market, By Region, 2021-2029 (USD Mllion)
18 Molecular Diagnostics Market, By Region, 2021-2029 (USD Mllion)
19 Coagulation Market, By Region, 2021-2029 (USD Mllion)
20 Microbiology Market, By Region, 2021-2029 (USD Mllion)
21 Others Market, By Region, 2021-2029 (USD Mllion)
22 Global In Vitro Diagnostics Quality Control Market: By Type Scope Key Takeaways
23 Global In Vitro Diagnostics Quality Control Market, By Type Segment: Revenue Growth Analysis
24 Quality Controls Market, By Region, 2021-2029 (USD Mllion)
25 Data Management Solutions Market, By Region, 2021-2029 (USD Mllion)
26 Quality Assurance Services Market, By Region, 2021-2029 (USD Mllion)
27 Others Market, By Region, 2021-2029 (USD Mllion)
28 Global In Vitro Diagnostics Quality Control Market: By End-use Scope Key Takeaways
29 Global In Vitro Diagnostics Quality Control Market, By End-use Segment: Revenue Growth Analysis
30 Home Care Market, By Region, 2021-2029 (USD Mllion)
31 Laboratory Market, By Region, 2021-2029 (USD Mllion)
32 Hospitals Market, By Region, 2021-2029 (USD Mllion)
33 Others Market, By Region, 2021-2029 (USD Mllion)
34 Regional Segment: Revenue Growth Analysis
35 Global In Vitro Diagnostics Quality Control Market: Regional Analysis
36 North America In Vitro Diagnostics Quality Control Market Overview
37 North America In Vitro Diagnostics Quality Control Market, By Application
38 North America In Vitro Diagnostics Quality Control Market, By Type
39 North America In Vitro Diagnostics Quality Control Market, By End-use
40 North America In Vitro Diagnostics Quality Control Market, By Country
41 U.S. In Vitro Diagnostics Quality Control Market, By Application
42 U.S. In Vitro Diagnostics Quality Control Market, By Type
43 U.S. In Vitro Diagnostics Quality Control Market, By End-use
44 Canada In Vitro Diagnostics Quality Control Market, By Application
45 Canada In Vitro Diagnostics Quality Control Market, By Type
46 Canada In Vitro Diagnostics Quality Control Market, By End-use
47 Mexico In Vitro Diagnostics Quality Control Market, By Application
48 Mexico In Vitro Diagnostics Quality Control Market, By Type
49 Mexico In Vitro Diagnostics Quality Control Market, By End-use
50 Four Quadrant Positioning Matrix
51 Company Market Share Analysis
52 Siemens Healthcare GmbH: Company Snapshot
53 Siemens Healthcare GmbH: SWOT Analysis
54 Siemens Healthcare GmbH: Geographic Presence
55 F. Hoffmann-La Roche Ltd.: Company Snapshot
56 F. Hoffmann-La Roche Ltd.: SWOT Analysis
57 F. Hoffmann-La Roche Ltd.: Geographic Presence
58 Alere, Inc.: Company Snapshot
59 Alere, Inc.: SWOT Analysis
60 Alere, Inc.: Geographic Presence
61 Abbott: Company Snapshot
62 Abbott: Swot Analysis
63 Abbott: Geographic Presence
64 Bio-Techne: Company Snapshot
65 Bio-Techne: SWOT Analysis
66 Bio-Techne: Geographic Presence
67 Hologic, Inc. (Gen-Probe): Company Snapshot
68 Hologic, Inc. (Gen-Probe): SWOT Analysis
69 Hologic, Inc. (Gen-Probe): Geographic Presence
70 Qiagen N.V. : Company Snapshot
71 Qiagen N.V. : SWOT Analysis
72 Qiagen N.V. : Geographic Presence
73 Bio-Rad Laboratories, Inc.: Company Snapshot
74 Bio-Rad Laboratories, Inc.: SWOT Analysis
75 Bio-Rad Laboratories, Inc.: Geographic Presence
76 Quidel Corp., Inc.: Company Snapshot
77 Quidel Corp., Inc.: SWOT Analysis
78 Quidel Corp., Inc.: Geographic Presence
79 BioMerieux, Inc.: Company Snapshot
80 BioMerieux, Inc.: SWOT Analysis
81 BioMerieux, Inc.: Geographic Presence
82 Other Companies: Company Snapshot
83 Other Companies: SWOT Analysis
84 Other Companies: Geographic Presence
The Global In Vitro Diagnostics Quality Control Market has been studied from the year 2019 till 2029. However, the CAGR provided in the report is from the year 2024 to 2029. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the In Vitro Diagnostics Quality Control Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Frequently Asked Questions
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS