
Risk-Based Monitoring Software Market Size, Trends, and Analysis - Forecasts to 2026 By Types (Enterprise RBM Software and Site RBM Software), By Type of Component (Software and Service), By Type of Delivery Mode (Web-based [On-demand], Licensed Enterprise [On-premise], Cloud-based [SaaS]), By Region (North America, Europe, Asia Pacific, MEA, and CSA), By End-User (Pharmaceutical & Biopharmaceutical Companies, CROs, Medical Device Companies, Other End Users); Vendor Landscape, and Company Market Share Analysis & Competitor Analysis
The global risk-based monitoring (RBM) software is a centralized product with a base platform to incorporate data from various clinical tests into productive and applicable analytics. Drivers such as increasing awareness and knowledge about the usage of software and its advantages among the individuals in the market is speeding up the growth in the market. Also, factors such as increasing knowledge about the availability of software for conducting clinical tests, encouragement for more funds/investment by the government and other organizations in improving the number of clinical tests, rising demand for time-efficient and cost-friendly software are some other factors propelling the market growth. The risk-based monitoring software market is enabling the researchers to improve their research base by enabling easy access to market information, growth data, recent market developments, technological improvements in the market, various products launch, improve the research analysis of clinical tests and know the emerging opportunities. It helps the researchers to efficiently manage the clinical tests and maintain data reliability.
By the category of types, the market is segmented into two, namely, the enterprise & site RBM software. The largest segment is the enterprise as compared to the site type. This software facilitates the researchers and the professionals to access the clinical test data at a centralized location, leading to its widespread demand among the end-users.
Software and services are the two major components of the market. Increasing expenditure on research and development, rising demand from client and customer base, improving figures of clinical tests are impelling the software component to hold the largest share in this market compared to the service component.
By delivery mode, the market can be classified into licensed, web-based and cloud-based. The largest shareholder of this segment is the web-based one as it provides more efficiency. It enables the end-users to have easy access to market information and clinical test data. It also facilitates time efficiency and a cost-friendly base to the end-users.
CROs, Biopharmaceutical firms, medical device manufacturers, are the major clients of this software. The dominant shareholders, ensuring their highest growth rate are the biopharmaceutical firms. The increased expenditure on research and development by these companies makes them the largest shareholders of this market.
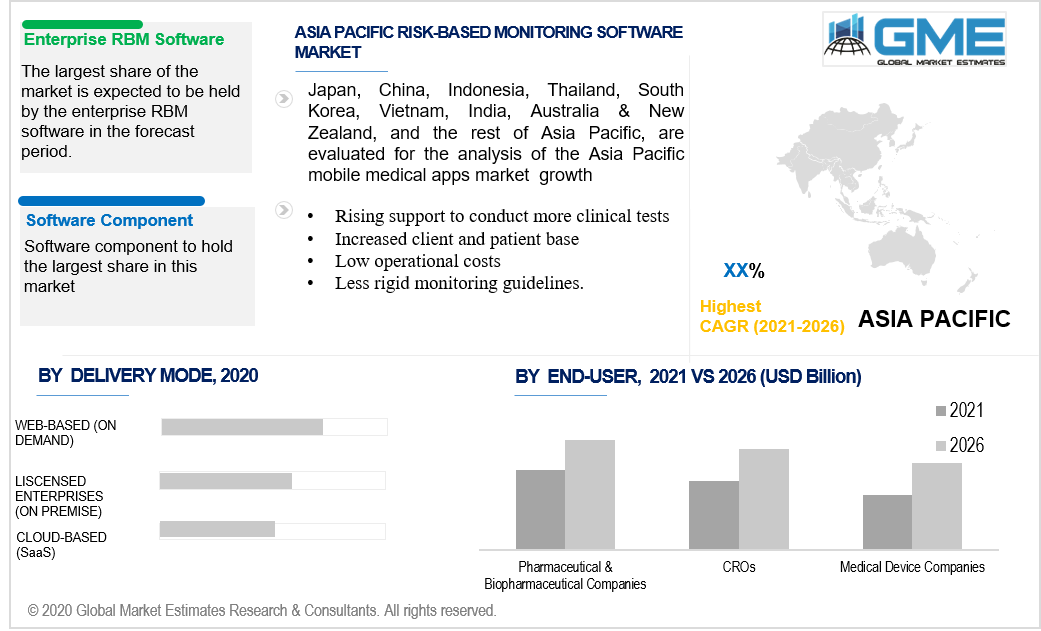
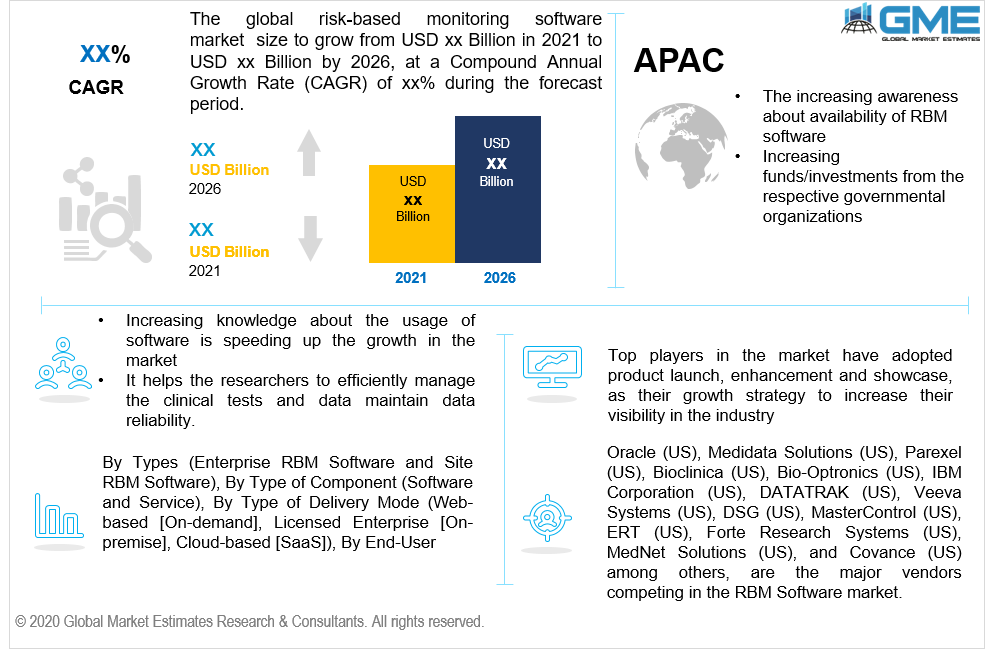
Based on the region, the dominant, as well as the largest share of this market, is held strong by U.S., and Canada as an entire North American region, however, the APAC region is expected to have the fastest growth. These growing regions are attributed to increasing funds/investments from the respective governmental organizations, rising support to conduct more clinical tests, increased client and patient base, low operational costs, and less rigid monitoring guidelines.
Medidata Solutions (US), Bioclinica (US), Parexel (US), IBM Corporation (US), DATATRAK (US), Oracle (US), Veeva Systems (US), DSG (US), MasterControl (US), Bio-Optronics (US), Forte Research Systems (US), ERT (US), MedNet Solutions (US), and Covance (US) among others, are the major vendors competing in the market.
Please note: This is not an exhaustive list of companies profiled in the report.
In October 2019, Parexel (U.S.), in partnership with Datavant, linked the real-world data of all the tests to boost the impact of the clinical study.
We value your investment and offer free customization with every report to fulfil your exact research needs.
The Global Risk-Based Monitoring Software Market has been studied from the year 2019 till 2026. However, the CAGR provided in the report is from the year 2021 to 2026. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply side analysis for the Risk-Based Monitoring Software Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the companies and customer analytics.

Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS