
Global Viral Inactivation Market Size, Trends & Analysis - Forecasts to 2026 By Method (Solvent Detergent Method, Pasteurization, Other Viral Inactivation Method), By Product (Kits & Reagents, Services, Viral Inactivation Systems &Accessories), By Application (Blood & Blood Products, Cellular & Gene Therapy Products, Stem Cell Products, Tissue & Tissue Products, Vaccines, and Therapeutics), By End User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Academic Research Institutes, Other End Users) By Region (North America, Asia Pacific, Europe, Central & South America, Middle East & Africa); End-User Landscape, Company Market Share Analysis, and Competitor Analysis
The method of viral inactivation has been designed to cater to the safety of the therapeutics products during the processing and production stage. The safe drug administration, development of vaccines, and blood products require the viral inactivation method to ensure the non-contaminated processing of such products. The launch of new drugs and other therapeutics products requires viral clearance from the regulatory bodies. The requirement of viral clearance leaves room for the method of viral inactivation and its growing necessity in the biopharmaceutical domain. Eurofins Scientific is a prominent player in the viral clearance domain. The laboratory caters to the manufacturing and development needs of the biological product. Viral Clearance acts as a risk mitigator in the biopharmaceutical industry by ensuring that the steps used in viral removal are proficient. At present, risk management strategies are being adopted to propagate the efficacy of viral inactivation.
The enhanced value of the market is also determined by the increasing rate of drug approvals and the development of new drugs. In 2018, 59 drugs were approved by the FDA among which 44 were specialty drugs.
As the cost of drug development rises, the need for virus removal in drug production becomes necessary. The guidelines put in place by the WHO to ensure plasma products are protected from viruses also have a positive impact on the use of viral inactivation procedures.
The importance of the market is viewed from the expansion of the pharmaceutical industry and its rising expenditure. The pharmaceutical industry is increasing its outsourcing capacity for its R&D activities, and also witnessing growing mergers and alliances among the industry for vaccine and therapeutic drug development. The development of new vaccines will also have potential uses of the viral inactivation treatment/methods.
The automation of work is necessary for all spaces and manufacturers of viral inactivation are developing an automated system to cater to the dynamic needs of the laboratory settings. The automated systems enable less operator involvement and can perform the ph virus inactivation. The benefits also range from patient safety to pH adjustment. The introduction of automation also leads to the reduced labor in the virus inactivation process. The market’s performance is also enhanced by the increasing research conducted by scientists to develop new therapies to combat the virus in the human cell. CRISPR is one enzyme that holds the potential to fight Ebola.
The various treatments in the past have gained significant momentum and have an established market in the viral inactivation domain. The technological advances in UV-C treatment in the past made the technique an important step to be considered for viral inactivation.
Another technology changing the viral inactivation space is the Cold Plasma technique. The technique is useful in inactivating the distinct animal, human, and plant viruses.
Manufacturers of the vaccines are developing novel investment strategies to cater to the vaccination needs of the nations. As most inactivated vaccines have profound use in fighting polio, dengue, and hepatitis, the investment plan to distribute vaccines across the globe will add to the market growth.
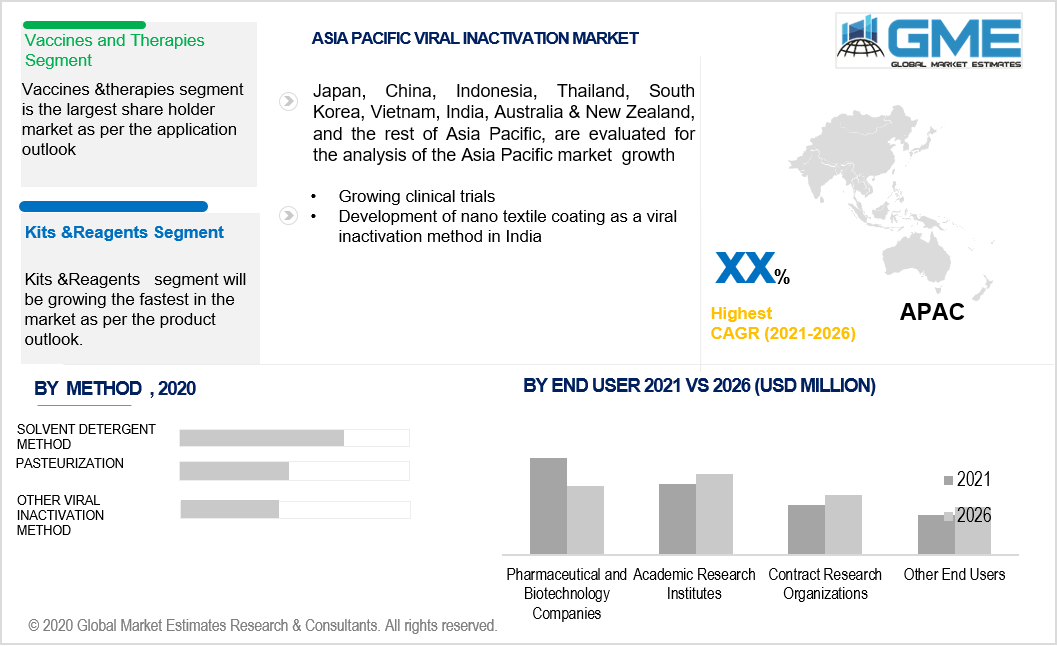
The market projections are also affected by the launch of the virus inactivation vaccines effective in fighting the novel virus. COVID-19 paved the way for the market to come up with solutions, kits & reagents, and inactivated vaccines to effectively fight the SARS-Cov-2. The COVDI-19 has led to a plethora of studies conducted and engage all startups, companies to invest in vaccines. IIT Madras startup in India developed the nano textile coating for inactivating the COVID-19 virus.
International conference on the safety of drugs, viral clearance, and testing also has positive benefits for the market growth.
The solvent detergent technique would occupy a dominant space in this market. As an established treatment/technique, solvent detergent in plasma-derived products has been applied for over 30 years. The technique’s advantages are widely propagated by clinical studies. The technique is considered safe and effective in the environment of congenital settings and bleeding disorders.
Kits and reagents will serve as a useful product in the market space owing to the product’s rising importance in the inactivation of the novel SARS virus. The manufacturers of the products are rapidly developing sample collecting kits and thoroughly investigating their efficacy. Companies like Lucence are maintaining a competitive edge in the kits and reagents domain.
The vaccines and therapeutics category will have a larger penetration owing to the large-scale development of the inactivated vaccines to treat dreadful diseases. Inactivated vaccines use the dead virus and bacteria to fight the diseases to create an immune response. To develop therapies and vaccines for the SARS-Cov-2, the use of inactivated vaccines was widely advocated and on a large scale. Such useful uses of virus inactivation in the development of vaccines would place the segment on a competitive front.
Pharmaceutical and biotechnology company’s dominant share in the market would be sustained by the growth of the sector across Asian countries, Europe, and the USA. The rise in generic drug production and the number of partnerships among biotechnology companies would also bear positively on the segment’s growth.
Academic &Research Institutes will bear a rising upward trend as universities across the globe are heavily participating in seminars, publishing journals, and research studies to develop more effective methods of viral inactivation.
North America’s assertive position is maintained by the region’s fast approval of drugs, rising collaboration among manufacturers of vaccines, and rising budget expenditure of the pharmaceutical companies. Countries like the USA are engaging heavily in clinical trials to propose the efficacy of the solvent detergent treatment. The Life science industry’s expanding business scale in the region also has a large bearing on the market valuations.
Asia Pacific’s strong leadership position would be supported by the rising level of research conducted in the viral inactivation domain. Manufacturers in India are rapidly developing inactivated vaccines to address the challenges of the novel COVID-19 virus. Region’s growing biotechnology sector and rising clinical trials would help to enhance the market growth.
Merck KGaA, Pall, Thermo Fisher Scientific, Sartorius, Bio Reliance, Clean Cells, Charles River, GE Healthcare, Rapid Labs, Bharat Biotech, and Danaher Corporation are key players operating in this market.
Please note: This is not an exhaustive list of companies profiled in the report.
We value your investment and offer free customization with every report to fulfil your exact research needs.
The Global Viral Inactivation Market has been studied from the year 2019 till 2026. However, the CAGR provided in the report is from the year 2021 to 2026. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Viral Inactivation Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS